⎯⎯⎯ About Us
Pioneering the future of eClinical technology with an AI-first approach to data capture and trial management.
Built for precision. Designed for regulation. Our platform meets the highest standards of data integrity.
Dedicated, isolated cloud environments for each customer—no data co-mingling, no forced upgrades, and infrastructure designed specifically for FDA- and EMA-regulated trials.
A modern, highly configurable EDC supporting no-code study builds, mid-study amendments without downtime, advanced edit checks, and robust audit trails.
Patient-centric modules for decentralized and hybrid trials, including eConsent and ePRO, designed to work on any device with multi-language and accessibility support.
Integrated RTSM for randomization and supply management, Captivate Coder for medical coding, and eTMF for audit-ready trial documentation across the lifecycle.
Expert services supporting implementation, validation, study configuration, and long-term operational success across the Captivate platform.
For more than a decade, Captivate has supported thousands of trials across phases, indications, and geographies—from emerging biotechs and device startups to global pharma, medtech, foundations, and academic groups.
Our approach is partnership-driven: we work as an extension of your team, aligning our configuration, services, and roadmap with your protocol, pipeline, and operational realities.
Captivate is a no-code required system with optional coding allowed for extra customizability. Our design tool allows you to build your forms and rules using the drag-and-drop build tool. This reduces the study set up time from months to weeks. Most teams get their studies up and running in just 4 weeks!
For more advanced design, you can use JavaScript to create custom forms such as clickable images and heatmaps, task-timing clocks, QR/barcode scanners, and more!

Risk Based Monitoring (RBM) supports both partial and targeted source data verification.
Study changes are both versatile and easy to manage. If a study amendment is required, a new CRF version can be assigned to specific sites or it can be made available automatically to all sites.
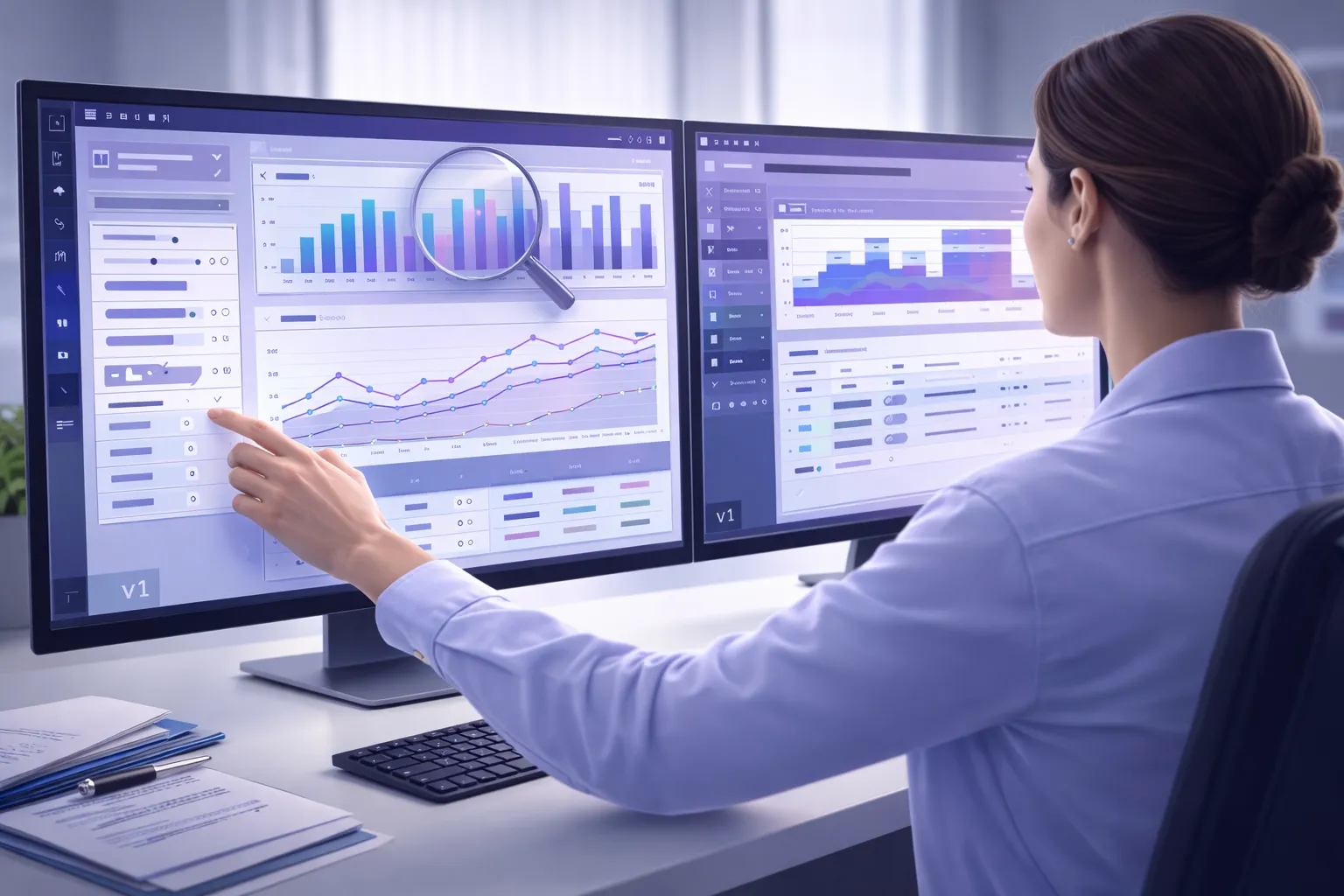
Interactive dashboards show your study progress in realtime and allow you to drill down on the data that matters.
Graphical, drag and drop interface. Cross study/site reporting: Key clinical data can be analyzed across studies and sites.
Users can explore and analyze clinical data on their own with ad hoc reports and dashboards.
Provides users up-to-date reports instantly, as their CRFs or their data change. Unlike other EDC systems, there are no additional steps or time wasted.
Users can access the tools from within the Captivate® Live Application.
Captivate is built on the belief that powerful clinical technology should be accessible, not exclusive. Through Weidley’s Wish, our flagship philanthropic program, we provide low- or no-cost access to enterprise-grade EDC and services for underfunded research in areas like rare disease, global health, and other high-impact, underserved conditions.
This combination of innovation, hands-on partnership, and social responsibility defines how we build, support, and continually evolve Captivate—for today’s trials and tomorrow’s breakthroughs.

Captivate is built on the belief that powerful clinical technology should be accessible, not exclusive. Through Weidley’s Wish, our flagship philanthropic program, we provide low- or no-cost access to enterprise-grade EDC and services for underfunded research in areas like rare disease, global health, and other high-impact, underserved conditions.
This combination of innovation, hands-on partnership, and social responsibility defines how we build, support, and continually evolve Captivate—for today’s trials and tomorrow’s breakthroughs.

Discover how Captivate can support your clinical research needs. Our team is here to answer questions and show you what’s possible.