⎯⎯⎯ Captivate RTSM
Flexible, Robust Randomization with Integrated Kit Tracking for Clinical Studies
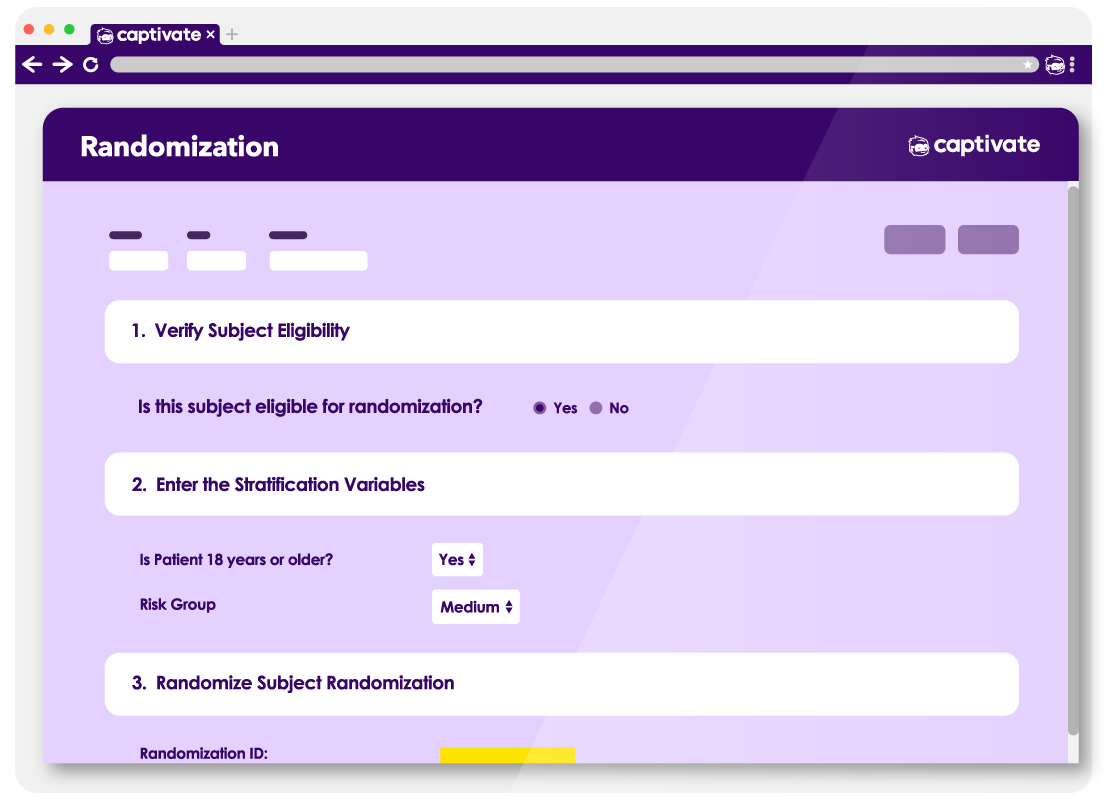

Captivate RTSM is a web-based Randomization and Trial Supply Management module designed to support a wide range of randomization strategies while maintaining operational simplicity, compliance, and audit readiness.
BBuilt for sponsors and CROs running interventional studies, Captivate RTSM delivers robust randomization capabilities without the overhead and rigidity of traditional IRT systems.
Designed to integrate seamlessly with the Captivate clinical data platform, Captivate RTSM enables study teams to configure and manage randomization schemes, maintain blinding, and track investigational kits within a single, unified environment.
Captivate RTSM supports multiple randomization methodologies to accommodate both simple and complex study designs.
Simple randomization.
Stratified randomization across protocol-defined factors
Permuted block randomization with configurable block sizes
Permuted block randomization with configurable block sizes.
Real-time enforcement of randomization rules at the point of assignment.
Logic managed centrally for consistent application across all sites and subjects.
Captivate RTSM includes built-in support for blinded study esigns while preserving participant safety and regulatory compliance.
Support for single-blind and double-blind study designs
Role-based access controls to protect blind integrity
Controlled emergency unblinding workflows
Full audit trails for all unblinding events

Captivate RTSM pairs randomization with structured kit ID setup and tracking to support investigational product oversight.
Centralized kit ID configuration
Assignment of kits based on randomization results.
Tracking of kit status throughout the study lifecycle.
Visibility into used, assigned, and available kits.
Captivate RTSM delivers intuitive workflows that support efficient site participation while maintaining centralized oversight.
Role-based access for sites, monitors, and central teams
Guided workflows for randomization and kit assignment
Immediate confirmation of randomization actions
Clear visibility into subject and kit history

Captivate RTSM operates within the broader Captivate ecosystem, enabling consistent study configuration and unified data oversight.
Shared subject and study context with Captivate EDC.
Unified audit trails across randomization, kits, and clinical data.
Simplified reporting and exports that include randomization metadata.
Reduced reconciliation effort across systems.

Captivate RTSM is designed to meet global regulatory expectations and support audits throughout the study lifecycle.
Compliance with 21 CFR Part 11, Annex 11, and applicable privacy requirements
Secure system access and data handling
Comprehensive audit trails for randomization, blinding, and kit events
Documented traceability for inspections and monitoring
Captivate RTSM delivers robust randomization and blinding capabilities without the cost, complexity, or long implementation timelines of full IRT platforms.
More flexible than rigid IRT systems that require extensive customization
More structured and auditable than manual or spreadsheet-based randomization
Designed to support study needs without unnecessary operational overhead
Captivate RTSM is used by sponsors and CROs running interventional clinical trials that require reliable randomization and controlled blinding.
Robust support for multiple randomization methods
Built-in blinding and emergency unblinding controls
Integrated kit tracking aligned with randomization outcomes
Unified workflows with Captivate EDC
Inspection-ready audit-ability without unnecessary complexity
Discover how Captivate can support your clinical research needs. Our team is here to answer questions and show you what’s possible.