Seamlessly Capture Data
Captivate® EDC
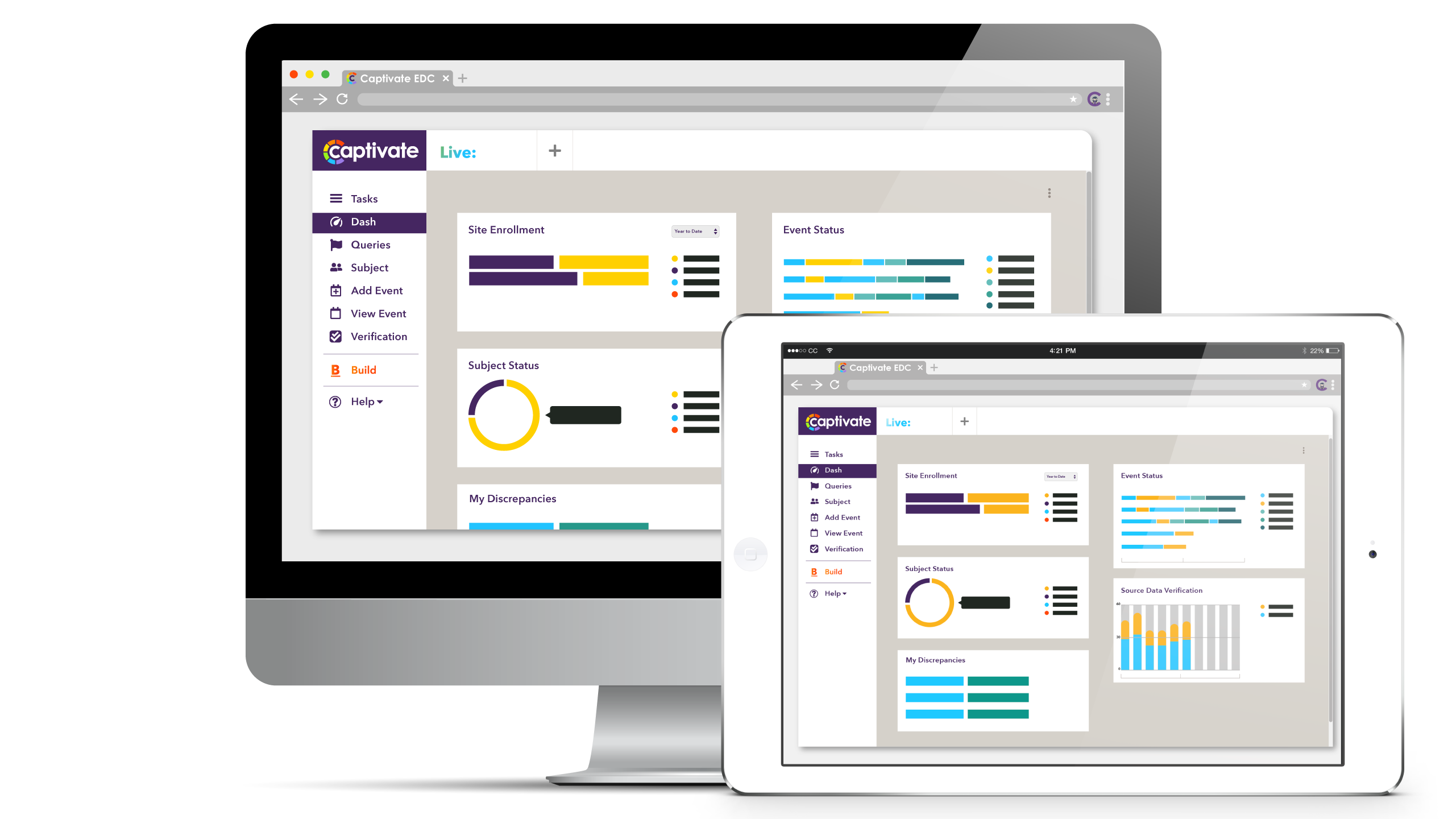
Captivate® EDC is the most dynamic and flexible system for collecting and managing clinical trial data on the market today. Built on REACT framework, Captivate® EDC offers a modern user interface and intuitive workflows for users.
The Captivate® Builder does not require any programming to design eCRFs or make mid-study changes which drastically reduces the time it takes to get a study up and running. The average build time in Captivate® EDC is just 4 weeks.
Although no programming is required, Captivate® EDC allows for custom coding to create complex rules or specialized fields such as clickable images, task-timing clocks, embedded videos, and more.
Captivate® Build
Captivate® Live
- Drag & drop builder that renders your design as you build
- Dynamic branching logic at the field & form level
- Form libraries including prebuilt industry standards
- Ability to clone & reuse any part of a CRF
- Role-specific restricted access fields for PHI/PII
- Item-level SDV, role-based workflows, & signature workflows
- Ability to configure queries & monitoring workflows
- Optional JavaScript field/form customization
- Large image/video uploads with built-in upload field
- Ability to set custom security controls
- Form versioning & multi-language capabilities
- Dynamic dashboards with real-time data displays
- Customizable roles & access/view permissions
- Risk-based Monitoring (RBM) & safety reporting
- Automated data imports & exports via SFTP
- In-application 21 CFR Part 11 compliant audit trail
- Accessibility features & searchable user guides
- Patient data & annotated CRF generation
- Test environments for seamless mid-study changes
- APIs for third-party system integrations
- Intuitive data entry workflows for site users
- Bulk-signing for SDV & PI signatures

