Trial Master File solution for effortless document management
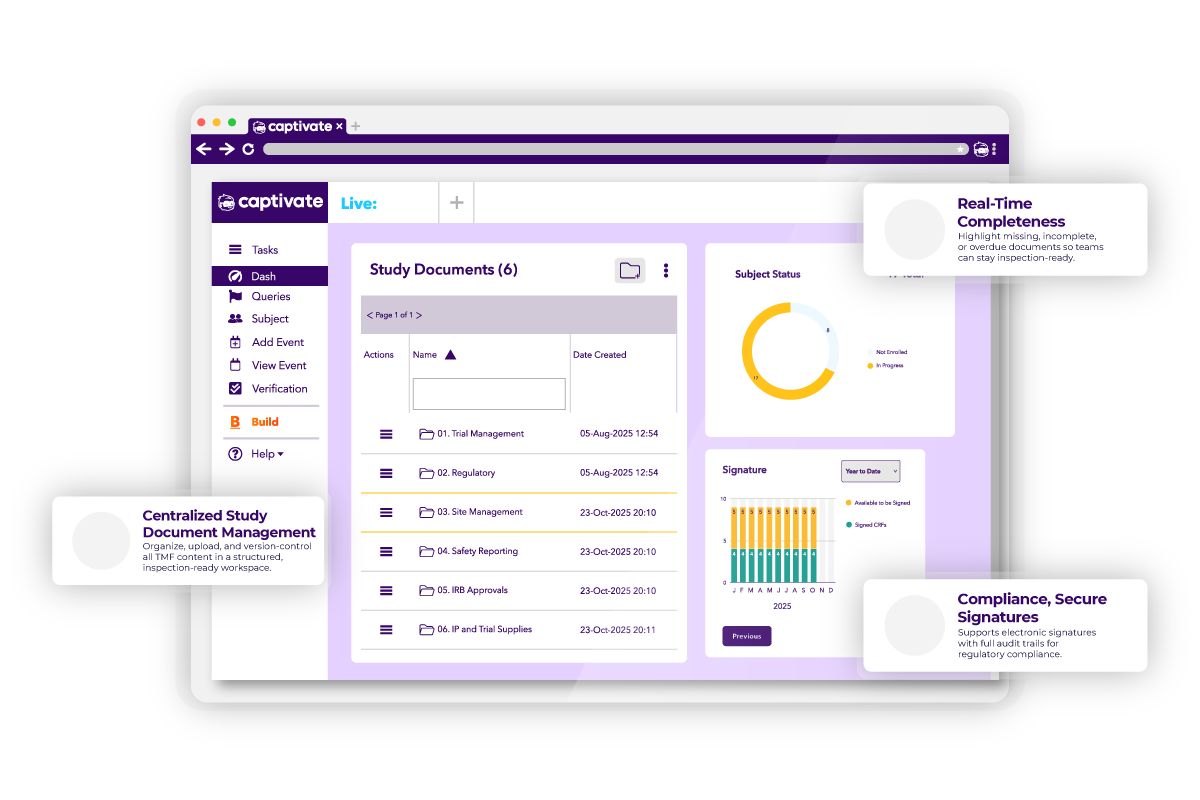
Captivate eTMF (Electronic Trial Master File) is designed to help studies and sponsors efficiently organize and manage clinical trial documentation, ensuring smooth submissions at the conclusion of the data collection cycle. Built on the robust Captivate platform, it simplifies the complexities of trial documentation while maintaining the highest standards of privacy, security, and scalability.