⎯⎯⎯ Captivate EDC
Flexible, Pre-Validated Electronic Data Capture Built for RealWorld Clinical Trials


Configure faster, stay inspection-ready, and scale seamlessly with a pre-validated EDC designed for studies of any size.
Captivate EDC is a modern, enterprise-grade Electronic Data Capture system designed for clinical data managers who need flexibility, control, and speed without the overhead of rigid, expensive platforms or the limitations of lightweight tools.
Built for studies ranging from small, fast-moving pilots to complex global programs, Captivate EDC combines intuitive, no-code study build tools with advanced optional customization and powerful livestudy capabilities.
Captivate EDC is pre-validated and compliant out of the box, helping teams reduce startup timelines and focus on running the study, not managing infrastructure or validation burden.
Captivate EDC is designed to support how data managers actually build and manage studies, balancing ease of use with advanced configurability.
Drag-and-drop CRF and visit design with conditional logic, validations, and calculations
Support for complex workflows, adaptive designs, and sub-studies
Reusable forms, visit schedules, and libraries to standardize across programs
Optional JavaScript support for advanced functionality
Full audit trails for all configuration and build changes
Most Captivate customers build studies using intuitive, drag-and-drop tools without writing code.
For more advanced use cases, Captivate also supports optional scripting and custom functions, giving experienced teams additional flexibility without forcing complexity on everyone.
Captivate EDC is pre-validated, meaning the core system has already undergone validation by our team. This significantly reduces the effort required by sponsors and CROs and accelerates time to golive.
Average study build time of approximately four weeks
Some simple studies live in as little as 24 hours
No need to perform full system validation, only study-specific UAT
Reduced documentation burden while maintaining inspection readiness

Captivate EDC is designed to sit at the center of your clinical data stack, supporting clean, controlled data flow across systems and vendors.
Native integration with Captivate VDC modules such as ePRO and eConsent
Secure bulk imports via SFTP for labs and external data sources
Configurable datasets for downstream analysis and reporting
Consistent audit-ability across integrated data sources
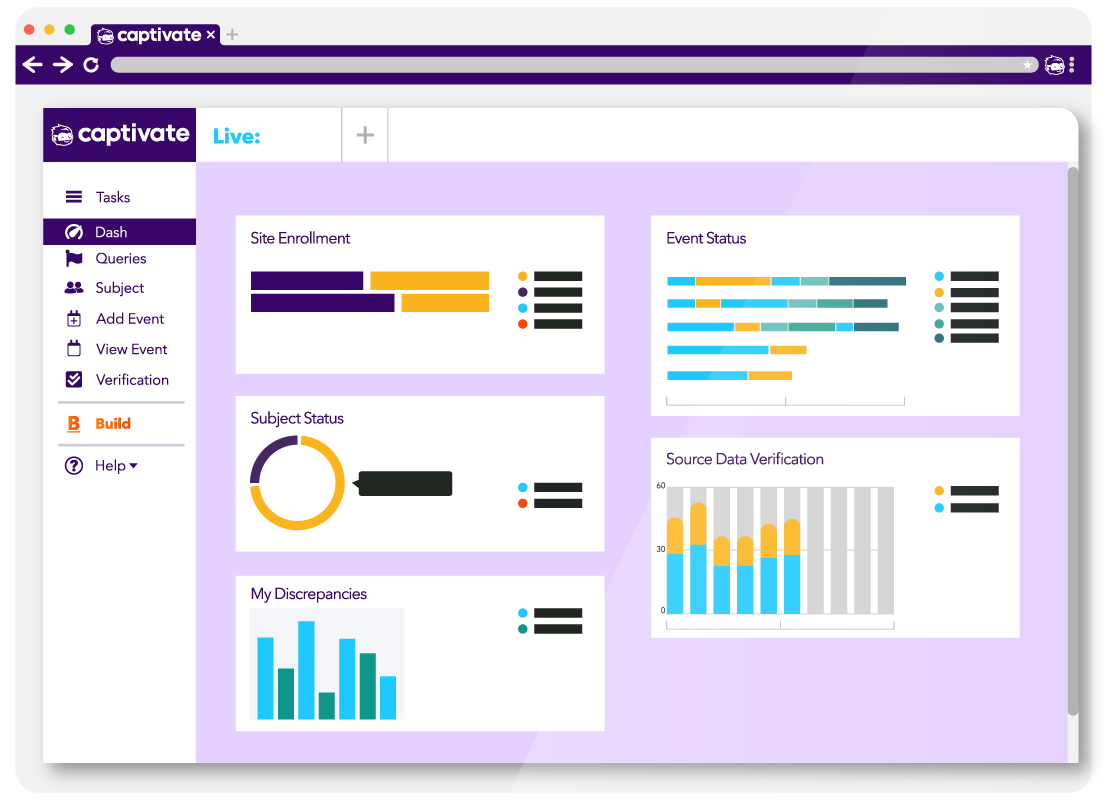
Once a study is live, Captivate EDC provides real-time visibility into study progress and data quality, enabling proactive monitoring and faster issue resolution.
Real-time dashboards showing enrollment, data completeness, and study health
Query management with configurable workflows
Role-based views for sites, monitors, sponsors, and data managers
Automated alerts and reminders to reduce manual follow-up

Captivate EDC maintains continuous traceability from first data entry through database lock.
Real-time access to current, clean data
Configurable exports including SAS-ready datasets
Comprehensive audit trails for subjects, users, roles, and data changes
Structured workflows for database lock and study closeout

Captivate EDC is compliant out of the box with global regulatory and privacy requirements and is designed to support audits and inspections without disruption.
Compliance with 21 CFR Part 11, Annex 11, GDPR, and HIPAA
Built-in PHI and PII masking features
Role-based access controls and authentication
Detailed audit trails for data, users, and permissions
Captivate EDC was built to avoid the tradeoffs teams often face when choosing between rigid
enterprise platforms and limited low-cost tools.
More flexible and faster to configure than legacy systems
More powerful and scalable than lightweight EDCs
Designed to grow with your pipeline without forcing migrations or disruptive upgrades
Captivate EDC is used by sponsors, CROs, academic institutions, diagnostics companies, and medical
device organizations running interventional trials and registries.
Faster study builds without sacrificing flexibility
Intuitive tools that reduce reliance on programming
Advanced customization when needed
Continuous inspection readiness
Responsive support from clinical data experts
Compliant out of the box
Discover how Captivate can support your clinical research needs. Our team is here to answer questions and show you what’s possible.