⎯⎯⎯ Captivate eTMF
Connected, Inspection-Ready Trial Master File Management
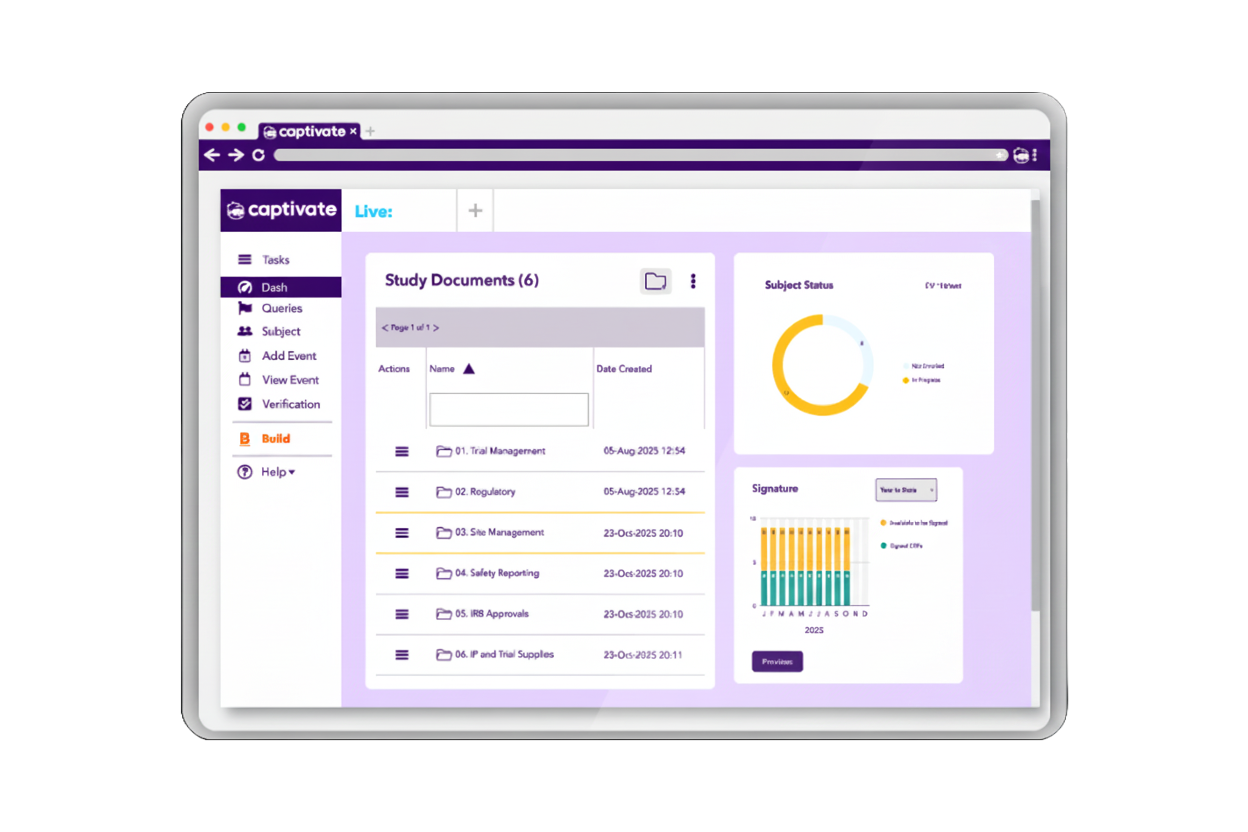


Captivate eTMF is a secure, standards-aligned electronic Trial Master File solution designed to help sponsors, CROs, and study teams manage essential trial documentation in a consistent, transparent, and inspection-ready manner.
Built to integrate with the broader Captivate clinical data platform, Captivate eTMF supports centralized oversight of regulatory and administrative artifacts while preserving traceability, compliance, and workflow flexibility.
Designed for real-world study operations, Captivate eTMF enables structured document organization, custom structure, automated lifecycle tracking, and controlled access helping teams reduce administrative burden, improve oversight, and support audits and inspections with confidence.
Captivate eTMF provides a framework for organizing and storing essential regulatory and operational documents throughout the clinical lifecycle.
Study documents are captured and indexed according to recognized industry standards to support consistency, clarity, and regulatory expectations.
Support for global TMF Reference Model indexing or custom taxonomy.
Consistent document metadata capture for reliable classification
Configurable folders, categories, and subcategories aligned to study needs.
Full search and filter capabilities across documents and metadata.
Structured organization ensures study content is accessible, traceable, and aligned with inspection readiness objectives.
Captivate eTMF tracks documents and their evolution through clear lifecycle states to maintain accountability and integrity.
Each document stored in Captivate eTMF includes a complete history of changes, versions, and actions.
Automated versioning with timestamped history
Audit trails for uploads, revisions, approvals, and access
Clear differentiation between draft, final, and archived content
Comprehensive version history supports compliance, reduces ambiguity, and simplifies audit responses.

Captivate eTMF supports structured processes for document creation, review, approval, and distribution while giving teams flexibility where protocols differ.
Study teams can define and automate document workflows that reflect their SOPs and operational cadence.
Configurable review and approval routing.
Alerts and reminders for pending actions.
Role-based access to review, approve, or annotate documents.
Support for collaborative review without version conflicts.
These workflows help reduce bottlenecks, ensure transparency, and drive accountability across functional teams.
Captivate eTMF is designed to meet regulatory and privacy expectations across global trials while supporting audits without unnecessary friction.
Compliance with 21 CFR Part 11, Annex 11, GDPR, and applicable privacy standards
Role-based access control with detailed permission levels
Secure document storage and transmission
Comprehensive audit trails for uploads, changes, approvals, and access log
Inspection-ready documentation and traceability support sponsors and CROs through regulatory submissions and inspections.

Captivate eTMF is integrated into the broader Captivate ecosystem, enabling aligned workflows across documents, clinical data, and operational artifacts.
Shared context with Captivate EDC and VDC modules for aligned workflows.
Centralized visibility into document status relative to clinical milestones.
Unified export capability for archived trial packages.
Consistent metadata and audit trails across systems.
This integration reduces the need for disconnected document filing systems and supports more coherent study oversight.

Completeness and review status across all studies
Search across studies, documents, and metadata seamlessly
Ready-to-use reports for operational leadership and QA
Support for multi-region regulatory requirements
Captivate eTMF is used by sponsors, CROs, and clinical operations teams responsible for regulatory and essential trial documentation.
Standards-aligned document organization and indexing
Comprehensive version tracking and audit trails
Configurable workflows that reflect real-world SOPs
Integrated clinical and operational context across the Captivate platform
Built-in compliance and inspection readiness
Discover how Captivate can support your clinical research needs. Our team is here to answer questions and show you what’s possible.